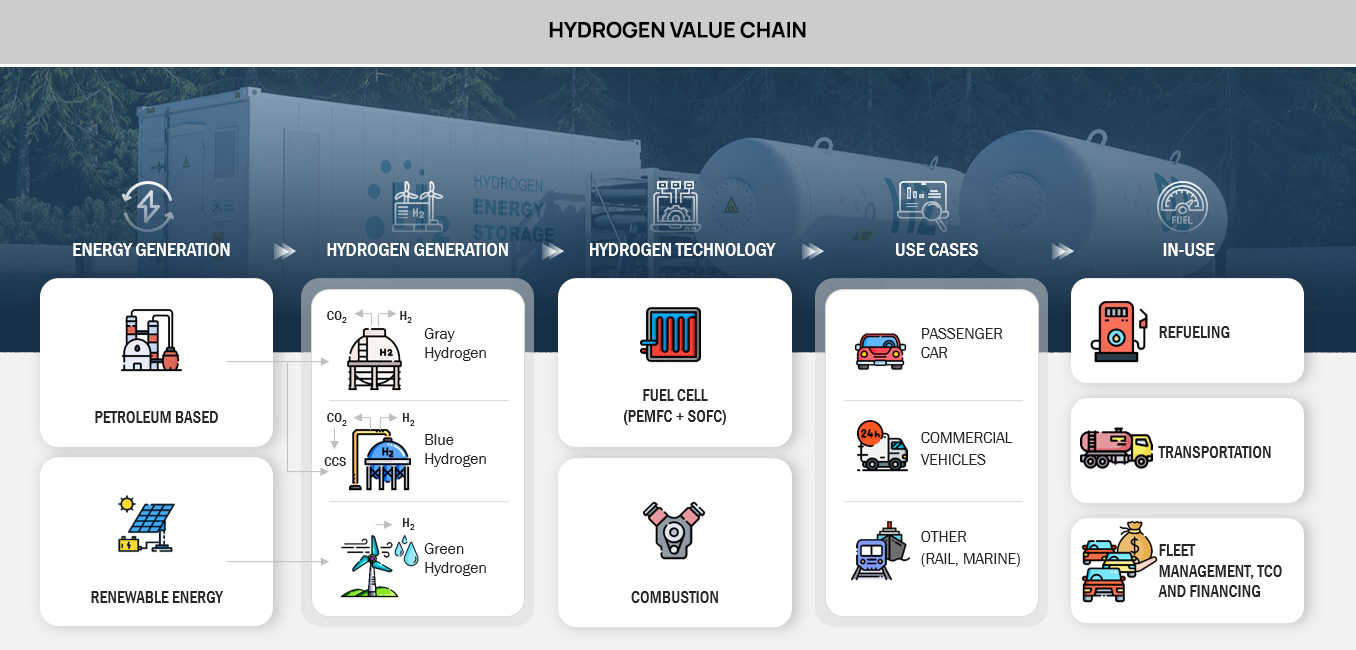
Hydrogen is a versatile and clean energy carrier that can be used in various applications. Here are some of the most commonly used hydrogen technologies & comparison of their advantages and disadvantages:
- Steam Methane Reforming (SMR): SMR is the most common method of hydrogen production, accounting for approximately 95% of global production.
- Electrolysis: Electrolysis is a process that uses electricity to split water into hydrogen and oxygen. There are two types of electrolysis: alkaline and proton exchange membrane (PEM).
- Biomass Gasification: Biomass gasification is a process that involves heating organic materials, such as wood or agricultural waste, in the absence of oxygen to produce a gas that can be purified into hydrogen.
- Nuclear: Nuclear hydrogen production involves splitting water using high-temperature nuclear reactors
- Photobiological: Photobiological hydrogen production involves using photosynthetic microorganisms, such as algae, to produce hydrogen.
- Solid Oxide Electrolysis Cells (SOEC): SOECs use a ceramic electrolyte to split water into hydrogen and oxygen. This technology is efficient, but it is still in the research and development phase.
- Thermochemical: Thermochemical hydrogen production involves using heat and chemical reactions to produce hydrogen from fossil fuels or other sources.
DOWNLOAD- https://www.marketsandmarkets.com/industry-practice/RequestForm.asp
Hydrogen storage technologies:
Hydrogen is considered a promising energy carrier for the future, and efficient storage methods are crucial for its widespread adoption. There are several hydrogen storage technologies, each with its own advantages and challenges. Here are some of the main hydrogen storage methods:
- Compressed Hydrogen (CH2):
- Description: Hydrogen gas is compressed at high pressures (typically 350-700 bar) and stored in tanks.
- Advantages: Relatively simple technology, widely used in industry.
- Challenges: Requires strong and heavy storage tanks, energy-intensive compression process.
- Liquid Hydrogen (LH2):
- Description: Hydrogen is cooled to extremely low temperatures (-253°C or -423°F) to become a liquid and stored in insulated tanks.
- Advantages: High energy density, commonly used in aerospace applications.
- Challenges: Energy-intensive liquefaction process, requires specialized and cryogenic storage infrastructure.
- Metal Hydride Storage:
- Description: Hydrogen is absorbed into a solid metal matrix to form metal hydrides.
- Advantages: High volumetric and gravimetric storage density, potentially safer than gaseous or liquid storage.
- Challenges: Limited reversibility (slow hydrogen release and uptake), can be heavy, and may have temperature constraints.
- Chemical Hydrogen Storage:
- Description: Hydrogen is stored in chemical compounds, and it is released through a chemical reaction.
- Advantages: Potential for high storage density, reversibility, and ease of transport.
- Challenges: Some chemical processes may require high temperatures, and the regeneration of the chemical compound may involve energy-intensive steps.
- Carbon-Based Materials (Hydrogen Sorption):
- Description: Hydrogen is adsorbed onto the surface of high-surface-area materials, such as activated carbon or carbon nanotubes.
- Advantages: Relatively lightweight, potentially reversible, and moderate operating conditions.
- Challenges: Limited volumetric and gravimetric density compared to other methods, adsorption and desorption kinetics can be slow.
- Liquefied Organic Hydrogen Carriers (LOHC):
- Description: Hydrogen is chemically bonded to a liquid organic compound, forming a carrier fluid.
- Advantages: Liquid at ambient conditions, potentially easier to handle and transport.
- Challenges: Energy-intensive for both hydrogenation and dehydrogenation, issues with system complexity and cost.
Applications of hydrogen storage technologies:
Hydrogen storage technologies have diverse applications across various sectors, driven by the need for clean and sustainable energy solutions. Here are some key applications of hydrogen storage technologies:
Transportation:
Fuel Cell Vehicles (FCVs): Hydrogen can be used as a fuel for fuel cells in vehicles, providing a clean and efficient alternative to traditional internal combustion engines. Compressed or liquid hydrogen storage is employed in fuel cell vehicles.
Renewable Energy Integration:
Grid Balancing and Energy Storage: Hydrogen can be produced during periods of excess renewable energy (e.g., from wind or solar power) and stored. The stored hydrogen can then be used to generate electricity during periods of low renewable energy availability, providing grid balancing and energy storage.
Industry:
Hydrogen as a Feedstock: Hydrogen is a crucial raw material for various industrial processes, such as ammonia production for fertilizers and the production of chemicals like methanol.
Hydrogen for Refining: Hydrogen is used in oil refining processes, such as hydrocracking and desulfurization, to produce cleaner fuels.
Backup Power and Remote Power Generation:
Backup Power Systems: Hydrogen fuel cells can provide backup power for critical infrastructure, such as data centers and hospitals, ensuring continuous operation during power outages.
Remote Power Generation: Hydrogen fuel cells can be used for off-grid power generation in remote areas where traditional power infrastructure is impractical.
Hydrogen production technologies:
This article explores the key advancements in hydrogen production technologies, shedding light on the methods that hold the greatest potential for a greener future.
- Steam Methane Reforming (SMR):
- Description: SMR is currently the most widely used method for hydrogen production, constituting a significant portion of the global hydrogen supply. It involves the reaction of methane (CH4) with steam (H2O) to produce hydrogen and carbon dioxide.
- Advantages: Well-established technology, cost-effective.
- Challenges: Produces carbon dioxide as a byproduct, contributing to greenhouse gas emissions.
- Electrolysis:
- Description: Electrolysis is a clean and efficient method that involves splitting water (H2O) into hydrogen and oxygen using an electric current. There are two main types: alkaline electrolysis and proton exchange membrane (PEM) electrolysis.
- Advantages: Can be powered by renewable energy, produces only oxygen as a byproduct.
- Challenges: Requires significant energy input, cost of electrolysis equipment.
- Photoelectrochemical (PEC) Hydrogen Production:
- Description: PEC hydrogen production combines the principles of photovoltaics and electrolysis. Semiconductor materials are used to absorb sunlight and directly split water into hydrogen and oxygen.
- Advantages: Solar-driven, potentially high efficiency.
- Challenges: Materials development, scale-up challenges.
- Biological Hydrogen Production:
- Description: Certain microorganisms, such as algae and bacteria, can produce hydrogen through biological processes. This can be achieved through photosynthesis or fermentation.
- Advantages: Renewable and environmentally friendly.
- Challenges: Low production rates, sensitivity to environmental conditions.
Hydrogen energy technology:
Hydrogen energy technology encompasses a broad spectrum of methods and applications aimed at harnessing hydrogen as a clean and efficient source of energy. As the world strives to reduce carbon emissions and transition to more sustainable energy systems, hydrogen is gaining increased attention for its potential role in various sectors. Here’s an overview of hydrogen energy technology:
- Production Technologies:
- In electrolysis, electrical energy is used to split water into hydrogen and oxygen.
- Although a widely used method, SMR produces hydrogen from natural gas and emits carbon dioxide.
- Storage Technologies:
- Hydrogen can be compressed and stored in high-pressure tanks. While effective, compression requires energy and results in some heat loss.
- Hydrogen can be cooled to very low temperatures to become a liquid, which allows for higher energy density during storage.
- Advanced materials, such as metal hydrides or chemical hydrogen carriers, are being explored for solid-state storage, offering potential advantages in terms of safety and efficiency.
- Transportation:
- : Hydrogen fuel cells convert hydrogen and oxygen into electricity, with water as the only byproduct.
- Hydrogen can be burned directly in internal combustion engines.
- Industrial Applications:
- Hydrogen is a crucial component in various industrial processes, including the production of chemicals such as ammonia and methanol.
- In oil refining, hydrogen is used to remove impurities from crude oil, contributing to cleaner fuel production.
- Power Generation:
- Hydrogen fuel cells can be used for distributed power generation, providing electricity and heat for residential, commercial, and industrial applications.
- Hydrogen can be used to store excess energy from renewable sources and later generate electricity when demand is high, contributing to grid stability.
- Research and Innovations:
- Ongoing research focuses on developing advanced materials for hydrogen production, storage, and transport, aiming to improve efficiency and reduce costs.
- The coupling of hydrogen technologies with renewable energy sources is a key focus, aiming to create a sustainable and interconnected energy ecosystem.
READ MORE- https://www.marketsandmarkets.com/industry-practice/hydrogen/hydrogen-technologies-comparison