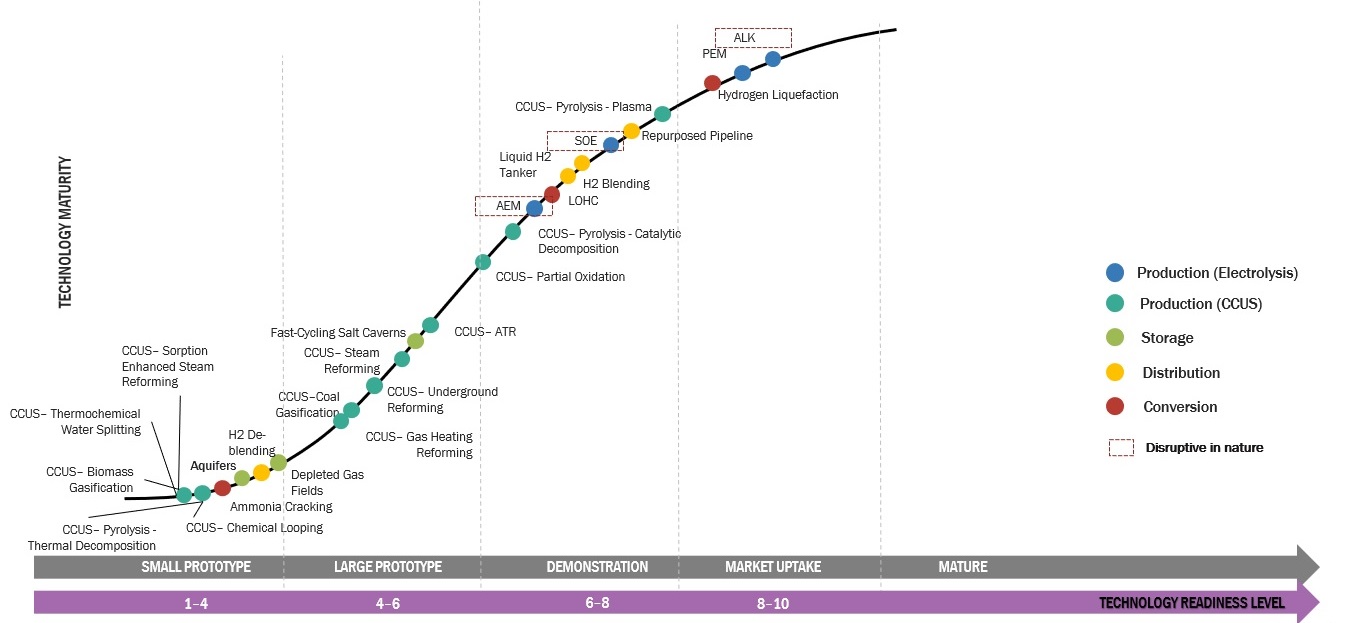
An essential component in assessing the sustainability and practicality of hydrogen as a clean energy source is the analysis of the costs associated with producing hydrogen. To propel progress in this area, a thorough analysis of the cost of producing hydrogen must be carried out. We can find areas for cost improvement by looking at several manufacturing techniques, like steam methane reforming or electrolysis, and evaluating the costs associated with each stage.
Hydrogen is regarded as a clean, adaptable, and efficient energy source, making it a promising energy carrier for the future. In contrast to traditional fossil fuels, hydrogen currently has a greater manufacturing cost. Reduced production costs are the result of doing Hydrogen Production Cost Analysis at every stage of the process, which is necessary to make hydrogen a cheaper energy source.
Download – https://www.marketsandmarkets.com/industry-practice/RequestForm.asp
The price of electricity, feedstock, plant capacity, technology type, and other factors all affect how much it costs to produce hydrogen.
Steam methane reforming (SMR), electrolysis, and coal gasification are the three main processes used to produce hydrogen.
1. Steam Methane Reforming (SMR): With SMR producing more than 75% of the hydrogen generated worldwide, it is now the most used technique of hydrogen production. The price of natural gas, the main feedstock used in SMR, affects the cost of producing hydrogen. Hydrogen and carbon dioxide are produced during the process by reacting natural gas with steam.
2. Electrolysis: Water is split into hydrogen and oxygen through a process called electrolysis, which uses electricity. Depending on the electrolysis method employed and the cost of power, the cost of producing hydrogen using electrolysis is determined. Alkaline and proton exchange membrane (PEM) technologies are the two main categories of electrolysis technology.
3. Coal Gasification: Coal is gasified by reacting it with oxygen and steam to create carbon monoxide and hydrogen. More hydrogen and carbon dioxide can be created by reacting the carbon monoxide and steam. The price of coal and the kind of technology employed have a significant impact on the cost of producing hydrogen through coal gasification.
The technique of Hydrogen Production Cost Analysis can utilize a number of strategies to make hydrogen production cost-effective. One strategy is to expand production volume since economies of scale make larger facilities more affordable. A different strategy is to boost production process efficiency, which can be done by utilizing cutting-edge technologies or optimizing existing procedures.
Hydrogen production from water
The process of drawing hydrogen gas (H2) from water molecules (H2O) is referred to as “Hydrogen Production from Water.” Usually, a variety of methods and tools are employed in this process to separate water into its component elements, hydrogen and oxygen. The most popular techniques for producing hydrogen from water are as follows:
1. Electrolysis: Water is electrolyzed, or broken down into hydrogen and oxygen, by running an electric current across it. Alkaline electrolysis and proton exchange membrane (PEM) electrolysis are the two primary forms of electrolysis. PEM electrolysis employs a solid polymer electrolyte, whereas alkaline electrolysis uses an alkaline solution.
2. Photolysis or Photocatalysis: This process directly splits water molecules into hydrogen and oxygen using light energy, most commonly from the sun. Photovoltaic cells or other materials that absorb light can be utilized to accomplish photolysis.
3. Thermochemical Processes: High-temperature reactions are used in several thermochemical processes to split water into hydrogen and oxygen. Heat from concentrated solar power or other high-temperature sources is frequently used in these operations.
4. Biological Processes: Certain microorganisms, including some kinds of bacteria and algae, can create hydrogen through biological processes. This could entail photosynthesis or fermentation.
Producing hydrogen from water with renewable energy sources can make the entire process environmentally benign. Hydrogen is regarded as a clean and adaptable energy carrier. It is noteworthy that the effectiveness and ecological consequences of hydrogen production are contingent upon the particular technique employed and the energy source supplying the process.
Water Hydrogen Generator
A Water Hydrogen Generator is a machine or system that uses the electrolysis process to create hydrogen gas (H2) from water. Utilizing an electric current, electrolysis divides water molecules (H2O) into hydrogen and oxygen. Typically, an electrolysis cell, electrodes, and an electrical power supply make up a water hydrogen generator.
A water hydrogen generator’s fundamental parts are as follows:
1. Electrolysis Cell: This is the chamber used for the electrolysis procedure. Electrodes are often composed of platinum or other conductive metals.
2. Electrodes: The electrodes are attached to an external power supply and submerged in water. By drawing in positively charged ions, or cations, the cathode facilitates the reduction of water to create hydrogen gas. The oxidation of water to produce oxygen gas is facilitated by the anode’s ability to attract negatively charged ions, or anions.
3. Electrical Power Source: Electrolysis requires an external power source, usually a direct current (DC) power supply, to supply the necessary electrical energy. The water-splitting reaction is started when an electric current is delivered to the electrodes.
4. Gas Separation and Collection: It is necessary to gather and separate the produced hydrogen and oxygen gasses. The gases are frequently gathered at the appropriate electrodes and then routed to different storage bins.
Water hydrogen generators are employed in many different contexts, such as small-scale hydrogen synthesis for research, teaching, or fuel cell powering in specialized uses. It’s important to remember that the system’s design, the materials utilized for the electrodes, and the water’s quality all affect how effective and useful water hydrogen generators are overall.
The purpose of an automobile hydrogen generator is to generate hydrogen gas (H2) within the vehicle, which can then be utilized as fuel in a hydrogen fuel cell. The fundamental idea is to use a chemical process, usually electrolysis, to make hydrogen, which is then fed into a fuel cell to produce energy to run the vehicle’s electric motor.
The main parts of an automobile hydrogen generator are as follows:
1. Electrolysis System: This section of the generator is responsible for splitting water into hydrogen and oxygen through the process of electrolysis. An electrical power source is needed to start the process, and the equipment usually comprises of an electrolysis cell with electrodes.
2. Water Supply: To make hydrogen through electrolysis, the generator requires a water source. To reduce contaminants and increase the electrolysis process’s efficiency, deionized or distilled water is frequently chosen.
3. Hydrogen Storage: The vehicle must be used to store the produced hydrogen gas safely. This frequently entails the use of high-pressure storage tanks or other on-board application-appropriate storage techniques.
4. Safety Systems: Safety elements including pressure relief valves, sensors, and other control systems are crucial to guarantee the safe operation of the hydrogen generator and storage system because hydrogen is a highly combustible material.
5. Integration with Fuel Cell: The vehicle’s hydrogen fuel cell receives the hydrogen that the generator produces. Hydrogen combines with atmospheric oxygen in the fuel cell to produce water and power as byproducts. The electric motor of the car is then powered by this electricity.
The field of hydrogen fuel cell vehicles, which aims to use hydrogen as a clean and sustainable energy carrier for transportation, includes hydrogen generators for cars. Several manufacturers and researchers are looking into ways to increase the overall viability of hydrogen fuel cell vehicles while also lowering prices, as the technology is still in its early phases of development and adoption.
Production of Green Hydrogen
The process of producing hydrogen gas (H2) in an environmentally sustainable way utilizing renewable energy sources is known as “Green Hydrogen Production.” In contrast to traditional techniques that depend on fossil fuels, green hydrogen production seeks to reduce or completely eradicate greenhouse gas emissions related to the production of hydrogen.
The electrolysis process, which splits water (H2O) into hydrogen and oxygen by running an electric current through it, is the most widely used technique for creating green hydrogen. Utilizing renewable energy sources to power the electrolysis process is a crucial component of producing hydrogen in a friendly manner. These sustainable resources consist of:
1. Solar Power: Solar panels generate power from sunlight, which can be utilized for hydrogen production through electrolysis.
2. Wind Power: Wind turbines use the kinetic energy of the wind to produce electricity. Through the process of electrolysis, hydrogen can be produced using this electricity.
3. Hydropower: Green hydrogen can be produced through electrolysis using water-moving electricity produced by dams or river currents.
The word “green” in “green hydrogen” refers to the process’s environmental friendliness because it uses renewable energy sources, emits no direct emissions while producing hydrogen, and helps reduce carbon footprints. Green hydrogen is becoming more and more recognized as a renewable energy source with potential uses in manufacturing, transportation, and energy storage, among other areas. Since it makes it possible to separate the generation of hydrogen from fossil fuels, it is seen as a crucial component in the shift to a low-carbon or carbon-neutral economy.
Offshore Hydrogen Production
Offshore hydrogen production is the process of producing hydrogen gas in facilities that are situated offshore, usually on platforms or other structures in maritime environments such coastal seas or the open ocean. In this idea, renewable energy sources, such as wind or marine currents, are harnessed to produce hydrogen using cutting-edge techniques like electrolysis.
Key components and considerations in Offshore Hydrogen Production include:
1. Renewable Energy Sources: Offshore facilities can produce power using renewable energy sources like marine energy systems (tidal or wave energy) or offshore wind farms. Hydrogen can then be produced via electrolysis or other techniques for hydrogen synthesis using this electricity.
2. Electrolysis: Water is split into hydrogen and oxygen through the process of electrolysis, which uses an electric current. Electrolysis devices on platforms driven by electricity supplied from offshore renewable sources may be used in offshore hydrogen generation.
3. Hydrogen Storage: It is necessary to store the created hydrogen before it is shipped to onshore facilities or consumed locally. Pipelines for transmission and undersea storage are two possible storage alternatives.
4. Platform Infrastructure: Infrastructure designed to endure marine conditions, such as platforms or buildings, is necessary for offshore hydrogen production facilities. These buildings might contain the hydrogen storage facilities, power generation systems, and electrolysis apparatus.
5. Transportation: Hydrogen can be produced and then transferred via pipelines, ships, or other modes of transportation to onshore facilities or straight to end users.
Using the abundant renewable energy resources found in maritime areas, offshore hydrogen generation is seen to be a potential strategy. Thanks to its clean and sustainable hydrogen source, it can help decarbonize a number of industries and sectors, such as transportation, industry, and energy generation.
The idea is in line with larger initiatives to create and apply green hydrogen production techniques that reduce environmental impact and facilitate the shift to a low-carbon or carbon-neutral economy.
Hydrogen Fuel Cell Generator
A Hydrogen Fuel Cell Generator is a device that uses a fuel cell, usually, to create energy through a chemical reaction between hydrogen and oxygen. This process, called electrochemical conversion, produces heat and water as byproducts in addition to electrical energy. Below is an explanation of the main elements and the underlying idea:
1. Fuel Cell: The fuel cell itself is the core component of the hydrogen fuel cell generator. Proton exchange membrane (PEM) and alkaline fuel cells are prevalent in hydrogen-powered applications, while there are other forms of fuel cells as well. For instance, in a PEM fuel cell, the anode receives hydrogen while the cathode receives oxygen from the surrounding atmosphere. Protons can flow between the anode and cathode thanks to the electrolyte in the center, producing both water and energy.
2. Hydrogen Supply: Hydrogen must be available for use as fuel in hydrogen fuel cells. There are a number of ways to get this hydrogen, such as compressed hydrogen gas tanks or, in certain situations, on-site production using methods like electrolysis.
3. Oxygen Supply: To finish the electrochemical reaction, the fuel cell requires an oxidizing agent, most commonly oxygen from the air. Fuel cells undertake a regulated electrochemical reaction to produce electricity, as opposed to burning hydrogen like combustion-based power plants do.
4. Electrical Output: Power is generated by the fuel cell’s hydrogen and oxygen reacting chemically. Electric motors in a variety of applications, including cars, portable generators, and backup power systems, can then be powered by this electrical energy.
5. Water and Heat Generation: Water is a byproduct of the electrochemical process. Steam is usually released as a consequence of the operation, which also generates heat. The pure water generated in many applications can be used or released without endangering the environment.
Particularly when the hydrogen is created using renewable energy sources (green hydrogen), hydrogen fuel cell generators are seen to be a clean and efficient method of generating electricity. In the pursuit of a low-carbon, more sustainable energy future, they are being investigated and used more frequently for a range of purposes, including as portable energy, stationary power generation, and transportation.
When hydrogen is created using renewable energy sources (green hydrogen), hydrogen fuel cell generators are thought to be an efficient and clean technique for generating electricity. In an attempt to move toward a more sustainable and low-carbon energy future, they are being investigated and progressively embraced for a range of uses, such as transportation, stationary power generating, and portable energy applications.
Typical processes for producing hydrogen fuel include:
1. Steam Methane Reforming (SMR): The most used industrial technique for producing hydrogen is this one. Methane (CH4) and steam (H2O) are reacted to produce carbon dioxide (CO2) and hydrogen gas. SMR is frequently used, but until carbon capture and storage (CCS) technologies are put in place, it is linked to carbon emissions.
2. Electrolysis: The most used industrial technique for producing hydrogen is this one. Methane (CH4) and steam (H2O) are reacted to produce carbon dioxide (CO2) and hydrogen gas. SMR is frequently used, but until carbon capture and storage (CCS) technologies are put in place, it is linked to carbon emissions.
3. Partial Oxidation of Hydrocarbons: Hydrocarbons and oxygen (O2) are reacted in this process to produce carbon monoxide (CO) and hydrogen gas. After that, more carbon dioxide and hydrogen can be created by processing the carbon monoxide.
4. Thermochemical Water Splitting: High temperatures are used in this method to split the chemical bonds in water, separating the hydrogen and oxygen. Concentrated solar power or other sources of high temperature heat can power it.
5. Biological Hydrogen Production: Hydrogen can be produced biologically by several microorganisms, including some bacteria and algae. That covers both photosynthesis and fermentation.
A number of factors, including cost, efficiency, and environmental concerns, influence the method choice. More attention is being paid to creating processes that use renewable energy sources to create hydrogen, which makes hydrogen a more sustainable and clean fuel choice. As was previously indicated, the term “green hydrogen” is frequently used to emphasize the ecologically benign character of hydrogen produced using renewable energy.
Read More – https://www.marketsandmarkets.com/industry-practice/hydrogen/hydrogen-production-cost-analysis