Navigating the Growth Trajectory
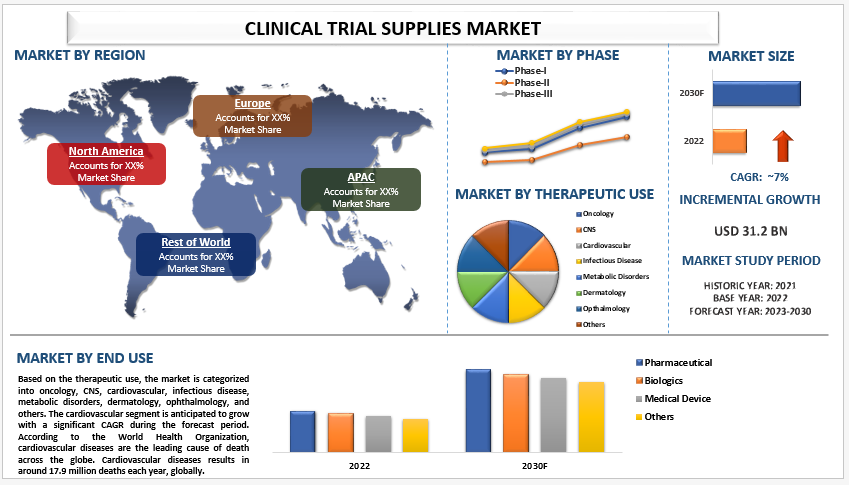
The Clinical Trial Supplies Market, valued at an impressive USD 31,277.69 million in 2021, is poised for remarkable growth, projected at a compelling Compound Annual Growth Rate (CAGR) of 7% from 2022 to 2030. This ascent is propelled by a confluence of factors, including augmented R&D expenditure by pharmaceutical giants, a surge in registered clinical trials, and the paradigm shift towards decentralized clinical trials.
Key Drivers of Market Expansion
1. Surge in R&D Expenditure
The pharmaceutical landscape has witnessed a seismic shift with a substantial surge in Research and Development (R&D) expenditure. In 2019 alone, the industry’s R&D investments peaked at a staggering 83 billion, marking a tenfold increase from the 1980s. The substantial financial injection into R&D has propelled the growth of the Clinical Trial Supplies Market, creating a fertile ground for innovation and discovery.
2. Decentralization Trend in Clinical Trials
A notable trend shaping the market is the decentralization of clinical trials. This transformative approach streamlines trial processes, fostering accessibility and participation. The shift towards decentralized trials is reshaping the dynamics of the clinical trial supplies landscape, optimizing logistics and enhancing efficiency.
Access Sample PDF Here- https://univdatos.com/get-a-free-sample-form-php/?product_id=40687
Major Players Paving the Way
Leading the charge in the Clinical Trial Supplies Market are major players such as Thermo Fisher Scientific; Catalent, Inc.; Parexel; Almac Group; Marken; Piramal Pharma Solutions; Inizio; Recipharm; Myonex; and Rubicon Research Pvt. Ltd. These industry titans have strategically engaged in mergers, acquisitions, and partnerships, enriching the market with cutting-edge technologies and innovative solutions.
Illuminating Market Dynamics and Insights
1. Therapeutic Use Dynamics: Cardiovascular Segment Takes Center Stage
The therapeutic use segment unfolds with the cardiovascular domain poised to witness significant growth during the forecast period. Cardiovascular diseases, claiming around 17.9 million lives annually globally, beckon for groundbreaking treatments. The demand for clinical trial supplies in the development of novel cardiovascular drugs propels the sector into the limelight.
2. Phase Insights: Phase-III Trials Dominate
In the phase delineation, Phase-III trials emerge as the dominant force in the market as of 2021. Despite the relatively low number of drugs tested in this phase, the intricate nature and complexity amplify the demand for an efficient supply chain and logistics system, driving market growth.
3. End-User Landscape: Pharmaceutical Sector Commands Significance
The pharmaceutical segment commands a significant share in the end-user landscape in 2021. The dominance is attributed to the surge in clinical trials conducted by pharmaceutical companies, especially focusing on chronic diseases like cancer, diabetes, and cardiovascular ailments. The burgeoning demand for clinical trial supplies in this realm propels sustained market growth.
4. Regional Growth: Asia Pacific Emerges as a Beacon
The Asia Pacific region emerges as a beacon of growth, anticipating rapid expansion in the coming years. Factors such as a burgeoning diabetic population, increasing healthcare expenditure, and robust collaborations between pharmaceutical companies and Contract Research Organizations (CROs) fuel the region’s prominence in the Clinical Trial Supplies Market.
Browse Research Methodology, Report Description & Table of Contents- https://univdatos.com/report/clinical-trial-supplies-market
Closing Thoughts
In conclusion, the Clinical Trial Supplies Market stands at the precipice of transformative growth, fueled by increased R&D investments, decentralized trial trends, and dynamic collaborations. The market’s journey towards innovation and efficacy promises a future where clinical trials are not only groundbreaking but also accessible on a global scale.